| PROPELLANT COMBUSTION CHARTS |
How To Use These Charts
For each of the propellant combinations shown above, four graphs have
been provided. These graphs can be used to estimate (1) the optimum
mixture ratio of the combustion reactants, (2) the adiabatic flame
temperature of the combustion reaction, (3) the average molecular weight
of the combustion products, and (4) the specific heat ratio of the
combustion products. This data is necessary to determine the velocity of
the exhaust gases expelled from a rocket engine, which in turn
determines the engine's thrust. Adiabatic flame temperature and gas
molecular weight have been calculated using the freeware program STANJAN.
Mixture Ratio is the ratio of oxidizer mass to fuel mass. We define
the optimum mixture ratio as that which will produce the highest
specific impulse for the given reactants. A propellant's optimum mixture
ratio is a function of the pressures at which the rocket engine will
operate. An engine with a high combustion chamber pressure and a low
nozzle exit pressure, i.e. a large section ratio, will have the highest
optimum mixture ratio.
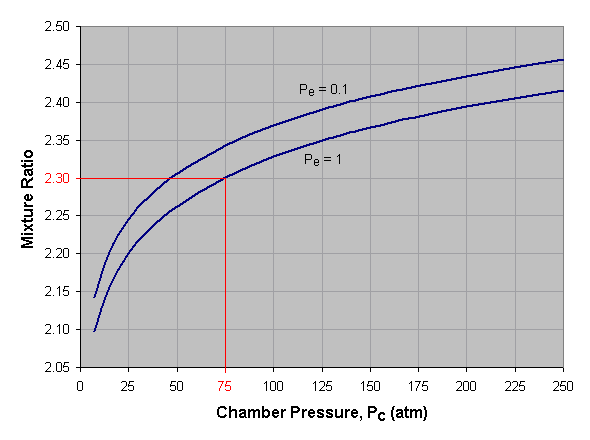
Below we see a graph of optimum mixture ratio versus combustion
chamber pressure for liquid oxygen and kerosene at two different nozzle
exit pressures (Pe). To use this graph, select the desired chamber
pressure across the bottom axis of the graph and draw a vertical line.
When the vertical line intersects the curve for the desired exit
pressure, draw a horizontal line to the left and read the corresponding
mixture ratio off the vertical axis of the graph. If an exit pressure
other than those shown is desired, estimate the position of the exit
pressure curve by interpolating between those given. For instance, the
curve for a Pe of 0.7 atmosphere lies approximately one-third the
distance from the Pe = 1.0 curve to the Pe = 0.1 curve. In the given
example we've selected a combustion chamber pressure of 75 atmospheres
and a nozzle exit pressure of 1 atmosphere, which gives us an optimum
mixture ratio of 2.30.
Adiabatic flame temperature is the temperature achieved by a
combustion reaction that takes place adiabatically, that is, with no
heat entering or leaving the system. It is the maximum temperature that
can be achieved for the given reactants and is used to estimate the
combustion chamber temperature (Tc) in a rocket engine.
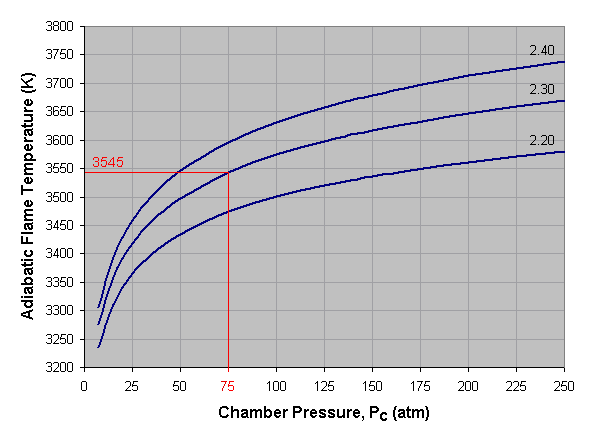
Below we see a graph of adiabatic flame temperature versus
combustion chamber pressure for liquid oxygen and kerosene at three
different mixture ratios. To use this graph, select the desired pressure
across the bottom axis of the graph and draw a vertical line. When the
vertical line intersects the curve for the desired mixture ratio, draw a
horizontal line to the left and read the corresponding adiabatic flame
temperature off the vertical axis of the graph. If a mixture ratio other
than those shown is used, estimate the position of the mixture ratio
curve by interpolating between those given. For instance, the curve for a
mixture ratio of 2.25 lies approximately midway between the 2.20 and
2.30 curves. In the given example we've selected a pressure of 75
atmospheres and a mixture ratio of 2.30, which gives us an adiabatic
flame temperature of approximately 3,545 Kelvin.
The exhaust gas molecular weight is the average molar weight of the
combustion products, that is, the mass of the exhaust gas divided by the
number of moles.
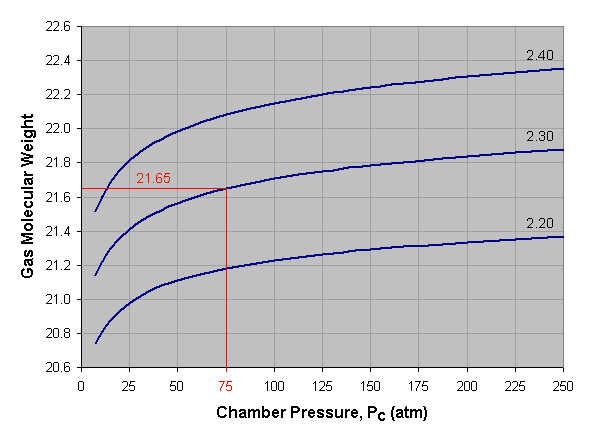
Below is a graph of gas molecular weight versus combustion
chamber pressure for liquid oxygen and kerosene at three different
mixture ratios. To use this graph, select the desired pressure across
the bottom axis of the graph and draw a vertical line. When the vertical
line intersects the curve for the desired mixture ratio, draw a
horizontal line to the left and read the corresponding gas molecular
weight off the vertical axis of the graph. For mixture ratios other than
those shown, estimate by interpolating between the given curves. In the
provided example we've selected a pressure of 75 atmospheres and a
mixture ratio of 2.30, which gives us an average gas molecular weight of
about 21.65.
The gas molecular weights shown below are taken at the combustion
chamber. The molecular weight will increase slightly as the gas expands
and cools while moving toward the nozzle exit.
Specific heat is the amount of heat necessary to raise the
temperature of one gram of a substance one degree Celsius. Specific heat
ratio is the ratio of constant-pressure specific heat to
constant-volume specific heat.
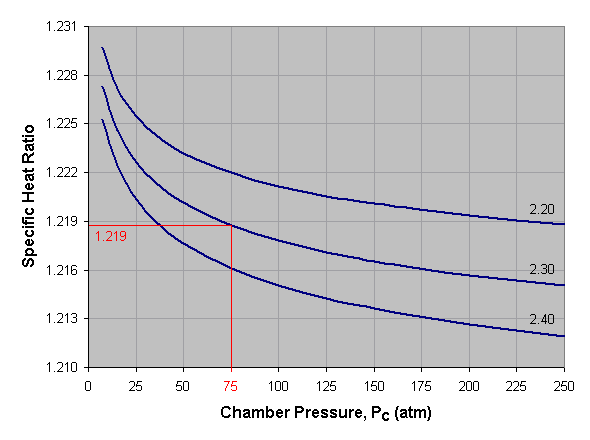
Below is a graph of specific heat ratio versus combustion chamber
pressure for liquid oxygen and kerosene at three different mixture
ratios. To use this graph, select the desired pressure across the bottom
axis of the graph and draw a vertical line. When the vertical line
intersects the curve for the desired mixture ratio, draw a horizontal
line to the left and read the corresponding specific heat ratio off the
vertical axis of the graph. For mixture ratios other than those shown,
estimate by interpolating between the given curves. In the provided
example we've selected a pressure of 75 atmospheres and a mixture ratio
of 2.30, which gives us a specific heat ratio of about 1.219.
The specific heat ratios shown below are taken at the combustion
chamber. As the gas moves toward the nozzle exit it will expand and
cool, thus increasing the specific heat ratio slightly. In practice, an
average value of specific heat ratio is used when calculating exhaust
gas velocity.
Written by Robert A. Braeunig, copyright 2005.
Tidak ada komentar:
Posting Komentar
ORANMG PINTAR UNTUK TAMBAH PENGETAHUAN PASTI BACA BLOG 'ROTE PINTAR'. TERNYATA 15 NEGARA ASING JUGA SENANG MEMBACA BLOG 'ROTE PINTAR' TERIMA KASIG KEPADA SEMUA PEMBACA BLOG 'ROTE PINTAR' DIMANA SAJA, KAPAN SAJA DAN OLEG SIAPA SAJA. NAMUN SAYA MOHON MAAF KARENA DALAM BEBERAPA HALAMAN DARI TIAP JUDUL TERDAPAT SAMBUNGAN KATA YANG KURANG SEMPURNA PADA SISI PALING KANAN DARI SETIAP HALAM TIDAK BERSAMBUNG BAIK SUKU KATANYA, OLEH KARENA ADA TERDAPAT EROR DI KOMPUTER SAAT MEMASUKKAN DATANYA KE BLOG SEHINGGA SEDIKIT TERGANGGU, DAN SAYA SENDIRI BELUM BISA MENGATASI EROR TERSEBUT, SEHINGGA PARA PEMBACA HARAP MAKLUM, NAMUN DIHARAPKAN BISA DAPAT MEMAHAMI PENGERTIANNYA SECARA UTUH. SEKALI LAGI MOHON MAAF DAN TERIMA KASIH BUAT SEMUA PEMBACA BLOG ROTE PINTAR, KIRANYA DATA-DATA BARU TERUS MENAMBAH ISI BLOG ROTE PINTAR SELANJUTNYA. DARI SAYA : Drs.Simon Arnold Julian Jacob-- Alamat : Jln.Jambon I/414J- Rt.10 - Rw.03 - KRICAK - JATIMULYO - JOGJAKARTA--INDONESIA-- HP.082135680644 - Email : saj_jacob1940@yahoo.co.id.com BLOG ROTE PINTAR : sajjacob.blogspot.com TERIMA KASIH BUAT SEMUA.